|
|
10th
International Inter University Scientific Meeting
Academy of Studenica
PERSPECTIVES
IN MELANOMA MANAGEMENT
& NANOTECHNOLOGY IN BIOMEDICINE
Organizers:
Institute of Oncology
Sremska Kamenica; Union of Cancer Prevention
Societies of Vojvodina, Novi Sad; Clinic of Oncology, Nis; Institute
for Oncology and Radiology of Serbia, Belgrade Center for Bioengineering,
Faculty of Mechanical Engineering, University of Belgrade
President:
Vladimir Baltic Vice-presidents: Zlata
Janjic, Radan Dzodic, Borislava Nikolin; Djuro Koruga
|
|
|
| |
MOLECULAR
BIOLOGY OF MELANOMA
BaltiŠ V.
Institute of Oncology
Sremska Kamenica, Clinic for internal oncology, Sremska Kamenica,
Serbia and Montenegro
|
|
| |
ABSTRACT
Melanoma
is a tumor of melanocytes that originate embryonically from neural
crest. Melanocytic tumors include a wide range of premalignant and
malignant tumors. Melanomas undergo discretely through five development
phases (normal melanocyte, dysplastic nevus, radial-growth phase,
vertical-growth phase, and metastatic melanoma). In all studies
dealing with melanoma tumorigenesis numerous genetic changes have
been found (Table 1).
Table 1. Major genetic changes in melanom
|
Gene
|
Chromosome
|
Mutation
frequency
|
|
CDKN2A/16
|
9p21
|
80%
of melanoma cell lines
40% of hereditary cases
|
|
CDKN2A/p19ARF
|
9p21
|
10-40%
sporadic cases
Altered concomitantly to 16p
|
|
CDK4
|
12q13
|
Rare
in hereditary and sporadic cases
|
|
PTEN/MMAC1
|
10q24
|
40%
of melanoma cell lines
|
|
APC-promoter
1A
|
5q21
|
13-17%
of melanoma cell lines
|
|
CTNNB1
|
|
13%
of melanoma cell lines
|
|
PITSLRE
|
1p36
|
Regulation
cell changes and apoptosis rare
|
|
ETS-1
|
11p23-24
|
Transcription
regulation factor 60% reduced in Mel/m
|
|
p53
|
17p13
|
overexpression
common; mutation very rare
|
|
ras
|
1p(N-ras)
11p25(Ha-ras)
12p(ki-ras)
|
10-25%
of cases (90% of ras mutation)
rare (10% of ras mutation)
very rare
|
|
bel-2
|
18q21.3
|
u
20% primary melanoma / sensitivity of CTH
|
|
NF-1
|
17q11
|
?
|
|
MUSOD
|
6q
|
60%-80%
prim. melanoma
|
|
hMSH2
hMLH1
hPMS1
hPMS2
|
2p16
3p21
2q32
7p22
|
5%-22%
|
|
Crucial early events in melanocytic transformation is mutation CDKN2A
which localization on chromosome 9p21. Protein (p16) encoded by
this gene is prototypical member of class of protein bind to cyclin
D and CDK 4; the latter two proteins together phosphylate the pRB
protein, a reaction critical to the promotion of cell cycle progression
at to CDK4 eliminate this function. The CDKN2A locus (9p21) encodes
two proteins; p16 and p19ARF,
which also inhibits cell progression. p16 may be a major genetic
contributor to inhered melanoma risk. Mutation in other genes (CDK4,
RB1) on the biochemical pathway rarely confer melanoma predisposition.
One of the most frequent regions of genetic alteration in melanomas
is chromosome 10q22-24 (PTEN/MMAC1 or TEP1). This gene encodes a
protein with protein tyrosine phosphates and cytoskeletal protein
domains that appears to function in regulating tyrosine phosphorylation
and cell adhesion. Also, p53, APC, beta-catenine, NF1 and 2, nm23,
and transcription factor ETS-1 have an important role later during
invasion and metastasisation of melanoma. Today, the main efforts
of researching have been focused to APC, beta-catenine (CTNNB1)
and MMR-gene. Hypermethylation in APC-promoter 1A gene and missense
mutations in beta-catenine gene cause dysregulation of WNT signaling
system in melanoma. Melanocyte transformation and progression of
melanoma are also significantly influenced by growth factors (and
growth factor receptors (bFGF, EGFR, HGFR, VGEF, TGF-beta2, TNF
alfa and beta, IL-1,2,6,8,10 and GCSF) and angiogenesis growth factors.
Clark has considered that growth, development and progression of
melanoma occur in five phases (normal melanocyte, dysplastic nevus,
radial-growth phase vertical-growth phase, metastatic melanoma).
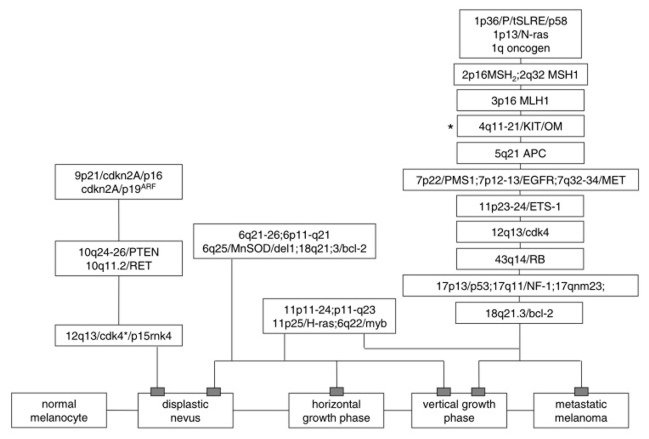
Based on all past molecular and genetic researches it is possible
to define the phases of melanoma initiation, progression and metastasisation
on a genetic model (Figure 1). In high-risk groups and hereditary
forms of melanoma it is possible to define genetic marker: CDKN2a,
p16, CDK4 or p19ARF, MMR-genes.
Genetic researches also contribute to the development of more recent
antimelanoma vaccines.
Figure 1. Model of genetic
changes during melanoma appearance and development
|
-
|
|

